Vaccinations
– The so called Covid-19 “vaccines” are actually synthetic gene therapy injections.
They employ synthetic, thermostable nucleotide sequences which are wrapped in a PEG (polyethylene glycol)-lipid nanoparticles to protect from destruction in the bloodstream and facilitate entry into the cells. The claim is that the cellular machinery will engage with these synthetic sequences and produce segments which code for the SarsCov2 S1 spike protein. It is believed that the immune system will mount a sufficient antibody response.
– Moderna even describes its technology as the “software of life,” not a vaccine.
– This synthetic gene therapy technology does not meet the definition of a traditional vaccine
– The WHO changed the definition of vaccination in the year 2020.
– Defining the Covid “Vaccinations” as vaccinations instead of gen therapy is so important because an EUA / Emergency Use Authorization issued on the basis of vaccination is much easier to obtain and requires fewer conditions, tests and regulations than an EUA issued on the basis of gene therapy.
This page about COVID-19 “Vaccinations” is absolutely overwhelmed with information, therefore please find additional COVID-19 ‘Vaccine” information after 9/22/2021 on our blog pages: Please find all COVID-19 vaccine related posts here.
– on 9/22/21 FDA authorizes booster dose of Pfizer-BioNTech COVID-19 Vaccine only for certain populations
– on 9/17/21 FDA advisory group rejects Covid boosters for most, limits the approval to people 65 and older and to high-risk groups
– on August 31, 2021 two of the top executives involved in vaccine research and testing at the Food and Drug Administration / FDA left the agency. They complained that the CDC had seized the right to make decisions.
– On August 23, 2020 The FDA and media declared that the FDA fully approved the Pfizer Covid-19 vaccine, which is -as always- only part of the truth.
Truth is that the FDA approved the manufacturing of Pfizer/BioNtech’s Comirnaty vaccine in Germany on 8/23/2021. It is the same vaccine like the Pfizer Covid-19 vaccine, but it is not yet available and it still requires 13 studies which will go on until 2027. Pfizer can be held liable for any adverse effects, if they administer this “approved” but not available vaccine. In Europe this Comirnaty vaccine by BioNtech Germany has only the “Conditional marketing authorization” which has been renewed on November 3, 2021 for one more year. The same is true for the AstraZeneca, Moderna and Janssen in Europe. EMA
On August 23, 2021 the FDA RE-ISSUED the EUA for the Pfizer Covid-19 vaccine, which has been administered since December 2020 in the USA. It still has the EUA and is in stock in huge amounts. Pfizer can not be held liable for this experimental vaccine. The FDA also signed an EUA Amendment on August 22, 2021 Ref: EUA 27034 extending the Pfizer COVID-19 Vaccine shelf-life from 6 months to 9 months.
In addition the booster shots will still just have the EUA, as well as the shots for children under the age of 16.
– According to USA Issuance of AuthorizationLetter to Pfizer it has not been approved, but is “an investigational vaccine not licensed for any indication.”
– FDA: “This product has not been approved or licensed by FDA.” Even after the EUA has been issued for this product, it still is being considered unapproved and still is under further investigation.
– The COVID-19 experimental synthetic gene therapy injections called “vaccine” has not been tested long term. For every other true vaccine scientists always needed between five to ten, or even more years to get the final approval. Of course it is possible to shorten the timeframe with an express approval process telescoping necessary approval steps, but nobody can telescope time or shorten the time to do long term tests. It just needs several years, 7 to sometimes 20 years to know if there are adverse effects in the future.
– This new technology has not only NOT been clinically tested long term but because of the rush, they also skipped animal testing. Actually they have been trying to get a mRNA “vaccine” to market for many years, but always failed with the animal trials, because the vaccinated animals died after they came in contact with the virus in the wild.
– The COVID-19 synthetic gene therapies Phase 3 trials are still ongoing, estimated completion date for Pfizer is January 31, 2023 and Moderna October 27, 2022.
Therefore the world is now in an experimental trial phase three – in the human experimental trial phase and they are administering it to millions of people.
– Pfizer recruited 43,661 volunteers (20,000 something received the placebo & other half the “vaccine”) & waited for people to come down with symptoms of Covid-19 & get a positive test (with the meaningless PCR test). Finally there were 170 people diagnosed with COVID-19. Of those 170, 162 had received a placebo shot & just 8 had received the real vaccine. So their 95% efficacy was based on just 170 cases. But in the FDA’s report on Pfizer’s vaccine, there were “3410 total cases of suspected, but unconfirmed COVID-19 in the overall study population, 1594 occurred in the vaccine group vs. 1816 in the placebo group.” These suspected cases can not be ignored just because there was no positive PCR test result, especially as we know that the PCR test can not detect an infection. Taking these cases into account the vaccine efficacy would be very low at 29%. BionTech also stated: “We may not be able to demonstrate sufficient efficacy in subsequent trials to obtain regulatory approval.” But the most critical point is that they based efficacy on the PCR test, which can not define the presence or absence of a clinical disease. Efficacy can only be determined by T-cell and antibody production following vaccination.
– Most people also believe that this would denote 95% reduction in hospitalizations or deaths, but in fact the 95% is calculated, based upon the “Primary Efficacy Endpoints”.
In the trial literature these endpoints are described by both companies as non-severe cold/flu SYMPTOMS coupled with a positive PCR. (which we learned is meaningless)
– These therapies do not prevent infection, merely reduction in one or more symptoms.
– Neither Moderna nor Pfizer claim that you develop immunity against COVID-19 nor that you can’t spread COVID-19 after receiving their COVID-19 “vaccines.” In fact, in their clinical trials, they specify that they will not even test for immunity. The trials are not designed to demonstrate a reduction in transmission, due to “operational realities”.
– The manufacturers also made it clear that efficacy beyond 2 months or so is unknown. So the 1% absolute risk reduction in mild/moderate, cold/flu symptoms may not last more than a few months.
– We know that the risk of people younger than 70 years, the COVID-19 infection fatality rates ranged from 0.00% to 0.31% with crude and corrected medians of 0.05%. So the risk is about the same as with every medium to sever flu, but the risk of taking the vaccine is mostly unknown.
– There is no reported Corona Virus in the injection people are getting.
It is a synthetic mRNA which makes a synthetic spike protein and this synthetic mRNA is not specific to the SARS-CoV2 virus. This synthetic mRNA has been patented, because a natural mRNA cannot be patented.
– Moderna, Pfizer/BioNTech and Arcturus Therapeutics COVID vaccines all employ an experimental messenger RNA (mRNA) using lipid nanoparticles (LNPs) that are “PEGylated,” meaning that the vaccine nanoparticles are coated with a synthetic and increasingly controversial PEG, which could potentially lead to life-threatening anaphylaxis.
– Pfizer excluded people with a history of severe allergic reactions from its clinical trials making the incidences of allergy or anaphylactic reactions unknown.
– Pfizer excluded pregnant women and children under the age of 16 from its clinical trials making the incidences of adverse effects unknown.
– The more disconcerting side effects are potential mid-long term effects due to the well-documented phenomena referred to as Antibody Dependent Enhancement (ADE) seen in some viruses such as coronaviruses. In previous vaccine trials the exposure of wild viruses to vaccine recipients resulted in severe disease, cytokine storms, and deaths in some animal and human trials. This is why we do not have a vaccine for the common cold, MERS or SARS.
– For the first time in history, the recipients’ cells will manufacture the pathogen, the mRNA stays in the cytoplasm of the cells, manufactures the S1 Spike Protein and then is destroyed. We only have a few months of data and do not know that these synthetic mRNAs last long enough to manufacture the protein but not long enough to exert deleterious effects, they may not be degraded immediately and perhaps linger for days, months, years. We just don’t know what will happen when we turn your bodies into a “viral protein factory”, thus keeping antibody production activated on a continual basis with no ability to shut down. Many leading scientists warn that there is also the potential for synthetic RNA to integrate into human DNA via the enzyme, reverse transcriptase. This may lead to mutagenesis, possibly cancer. It may lead to birth defects if it integrates into the germ cells of the injected. As the recipients’ cells are now producing the viral spike proteins, there is the potential for explosion of auto-immune diseases in coming years and there are concerns that the potential for antibodies against Syncytin-1 proteins (part of the placenta) may result in permanent infertility in women and possibly men as well, or the spike protein could cause inflammation, clot formation and heart attacks in the recipients who previously were exposed to SarsCov2.
Because there have been no long term tests, we just don’t know if nothing, some or all of this might be the case.
– Pfizer and Moderna are no longer recruiting people for their clinical trials.
So the additional information they need from large numbers of individuals will undoubtedly come from the millions of people who are being vaccinated right now. Which means that the world population has been enrolled as unwilling participants in clinical research study and experiments without informed consent.
– Every medication, vaccination needs the informed consent of the recipient, otherwise everyone handling this is in direct violation of The Nuremberg Code, a violation of article 6 of the UNESCO 2005 Universal Declaration On Bioethics And Human Rights, the Helsinki Declaration and many others. Also any coerced, forced & mandatory vaccinations are in direct violation of The Nuremberg Code and the Universal Declaration On Bioethics And Human Rights. If the potential trial subject is not relayed information about the absolute risk reduction in symptoms, and potential side effects, including ADE as well as efficacious alternatives for treatment or does not comprehend the information, it is a blatant violation of the Nuremberg code.
In addition federal law prohibits employers and others from requiring vaccination with a Covid-19 vaccine distributed under an EUA.
– Every pharmaceutical company and services producing, providing and administering COVID-19 vaccines have been relieved from liability and demanding any kind of compensation for adverse effects will be extremely difficult.
– The USA provides VAERS, a well established PASSIVE reporting system for adverse events. It is not very well known, and often even doctors do not know about its existence. So it is not used often. A research study by Harvard University initiated to create a new active system, concluded years ago that fewer than 1% of all cases are reported to the VAERS system. In mid February the VAERS already listed 602 deaths (CDC said 934) and ca. 39,000 adverse effects for the timeframe of December 2020 to mid of February 2021. VAERS listed 16,122 deaths, and 752,801 Adverse Events on September 24 2021 , with ca 392 Million COVID-19 vaccine doses administered up to September 30, 2021. Most of the deaths occurred 15 minutes to 48 hours after getting the vaccine. In addition the Pharma Industry is recommending the V-Safe App to every vaccinated person and it is highly questionable whether reactions reported via the app will end up in VAERS. There is also the issue, that neither healthcare nor life insurances will pay for treatment or deaths declared as an vaccine adverse event. So when healthcare bills due to an illness or death after the vaccine need to be paid any victim or family member has no incentive to file a report. Plus the numbers of adverse events in Europe are much higher, although the number of vaccinated people is similar. Considering the above issues the US numbers are very questionable and most be significantly higher!
In the EU’s vaccine adverse event reporting system EudraVigilance there were 26,523 deaths and 2,755,935 Covid Vaccine Adverse Events listed in the EU reporting system EudraVigilance with 1,737,150 cases which have not been recovered in EU as of October 2, 2021 with ca 565 Million COVID-19 vaccine doses administered up to September 30, 2021.
Both systems, the VAERS System in the USA and the EudraVigilance in EU System have been set up by US and EU authorities to be able to do research and check on adverse effects of medications and vaccinations. The EUA for the Covid vaccines even includes a stipulation, that the Pharma companies have to use VAERS and conduct appropriate research on adverse effects. This investigations are not being done and some judges in Germany even denied autopsies of victims who died shortly after the vaccination. So if systems exist to collect these data, why are those information not being used to do research about the adverse effects of those “vaccinations”? WHY?
Detailed information about the numbers and updates:
– Adverse events / Numbers and deaths
– Adverse Events in Europe
– Adverse Events regarding BionTech/Pfizer in Europe
– Adverse Events regarding Moderna in Europe
– Adverse Events regarding AstraZeneca in Europe
– Adverse Events regarding Janssen in Europe
So the ‘vaccine’ is still an experimental treatment, seems to be meaningless to combat COVID-19, but brings a huge unknown risk of adverse effects and deaths
February 1, 2022
Since the information about Covid-19 vaccine exploded, this page couldn’t hold more links and information. Therefore we are adding all other information about the vaccinations to our blog.
November 3, 2021
The European Medicines Agency EMA renewed the “Conditional marketing authorisation” for Comirnaty by BioNTech, Vaxzevria by AstraZeneca, Spikevax by Modern und Janssen Vaccine by Janssen on November 3, 2021 for one more year.
EMA
September 17, 2021
A panel of outside advisers to the U.S. Food and Drug Administration/ FDA on Friday, September 17, 2021 voted against approval of a booster dose of the Pfizer Inc (PFE.N)/BioNTech SE COVID-19 vaccine for people aged 16 and older.
An influential federal advisory panel overwhelmingly rejected a plan to offer Pfizer booster shots against COVID-19 to most Americans.
The surprising vote by the committee of outside experts assembled by the Food and Drug Administration/FDA was 16-2, with members expressing frustration that Pfizer had provided little data on the safety of extra doses. Many also raised doubts about the value of mass boosters, rather than ones targeted to specific groups.
“I don’t think a booster dose is going to significantly contribute to controlling the pandemic,” said Dr. Cody Meissner of Tufts University.
Meissner said he is worried about extra doses for younger age groups given the risk of heart inflammation that has been seen in mostly younger men after a second dose.
The Advisory Committee recommended against a COVID-19 vaccine booster shot for individuals 16 years and older in a 16 to 2 vote, but unanimously recommend the booster in individuals 65 years and older and those at high risk of severe COVID-19.
On September 22, 2021 the FDA authorized the booster dose of Pfizer-BioNTech COVID-19 Vaccine only for certain populations
August 31, 2021
Marion Gruber, Phil Krause, FDA’s top vaccine regulators, stepped down.
Two of the top executives involved in vaccine research and testing at the Food and Drug Administration / FDA left the agency. Marion Gruber and Phil Krause were director and deputy director, respectively, of the agency’s Office of Vaccines Research & Review.
The the pair complained that the CDC, and specifically its Advisory Committee on Immunization (ACIP), had seized the right to make decisions that had previously been left up to the FDA. The researchers were also supposedly upset with Marks for not standing up for them against the CDC. The Biden administration’s decision to announce a third round of mRNA “booster” shots without consulting them was merely the last straw.
Pain2 Power
RT
August 23, 2021
Biologics License Application (BLA) Approval of COMIRNATY to BioNTech Manufacturing GmbH, Mainz, Germany
NOT yet manufactured!
(needs 13 studies/ final report submission between 2023 to May 2027)
August 23, 2021
Re-issuance of the EUA for Pfizer Covid-19 Vaccine to Pfizer Inc, Collegeville, PA / USA
August 23, 2021
Explanation regarding the Biologics License Application (BLA) Approval of COMIRNATY and the Re-issuance of the EUA for Pfizer Covid-19 Vaccine.
August 22, 2021
On August 22, 2021 the FDA amended the EUA for the Pfizer Covid-19 Vaccine extending the shelf-live for Pfizer Cover-19 Vaccine from 6 to 9 months.
FDA – EUA amendment for Pfizer Covid-19 Vaccine on 8/22/2021
July 8, 2021
Joint CDC and FDA Statement on Vaccine Boosters:
Americans who have been fully vaccinated do not need a booster shot at this time.
CDC
PFIZER-BIONTECH COVID-19 VACCINE
BNT162B2 / sold under the brand name Comirnaty
“The Pfizer-BioNTech COVID-19 Vaccine is a vaccine and may prevent you from getting COVID-19. There is no U.S. Food and Drug Administration (FDA) approved vaccine to prevent COVID-19.”
http://labeling.pfizer.com/ShowLabeling.aspx?id=14472&format=pdf
https://www.pfizer.com/products/product-detail/pfizer-biontech-covid-19-vaccine
The Moderna COVID‑19 Vaccine
“The Moderna COVID-19 Vaccine is an unapproved vaccine that may prevent COVID-19. There is no FDA-approved vaccine to prevent COVID-19.”
https://www.modernatx.com/covid19vaccine-eua/recipients/
mRNA COVID-19 vaccines aren’t vaccines in the medical and legal definition of a vaccine.
What’s a Vaccine?
What makes an injection a vaccine?
Criteria for a vaccine:
In order to make an injection a vaccine:
1. we have to develop antibody to make us immune to the virus or bacteria. This development of the antibody is what makes a vaccine effective.
2. we need to be protected from getting a viral or bacterial infection.
3. the injection needs to reduce the number of deaths from that virus or bacterial infection
4. the injection has to help reduce the circulation of the virus or the bacteria against which we are vaccinating children and adults
5. we must have this injection reducing the transmission or spread of the virus or bacterial infection against we vaccinate from one person to the next
What kind of technology is in the COVID-19 injection?
It is an mRNA technology, which never before has been successfully used to make vaccines and has no track record that mRNA technology will function like a vaccine
1. we have no evidence that a mRNA injection will provide antibody immunity
2. we have no evidence that a mRNA injection will offer protection from getting a viral or bacterial infection.
3. we have no evidence that a mRNA injection will reduce the number of deaths from a virus or bacterial infection
4. we have no evidence whether a mRNA injection will reduce the circulation of a virus or bacteria against which we vaccinate
5. we have no evidence whether a mRNA injection will reduce the transmission or spread of the virus or bacterial infection from one person to the next.
We have a mRNA technology, but we do not have any evidence that this mRNA technology has been found to work the way we need a vaccine to work.
There is no reported Corona Virus in the injection people are getting.
It is a synthetic mRNA which makes a synthetic spike protein and this synthetic mRNA is not specific to the SARS-CoV2 virus.
This synthetic mRNA has been patented, because a natural mRNA cannot be patented.
So is the COVID-19 “Vaccine” a vaccine? NO
1. Does the COVID-19 “Vaccine” provide antibody immunity to the SARS CoV2 virus making it an effective vaccine? NO
Even the manufacturers say “We may not develop immunity to the SARS CoV2 virus with this injection”.
2. Does the COVID-19 “Vaccine” provide protection from getting SARS CoV2 viral infection? NO
The manufacturers and experts already said “If you get the injection it may reduce the symptoms, but it will not protect you from getting the SARS CoV2 viral infection.
3. Has the COVID-19 injection shown to reduce the number of deaths from a SARS CoV2 infection? NO
Not only that, but the manufacturers did not test whether this COVID-19 injection would reduce the number of deaths from a SARS CoV2 infection.
4. Has the COVID-19 injection shown to reduce the circulation of the SARS CoV2 virus in the population? NO
5. Has the COVID-19 injection shown to reduce the transmission or spread of the SARS CoV2 virus from one person to the next? NO
The manufacturers and scientific experts already said: “We did not test this injection to see if it would stop the transmission of the SARS CoV2 virus from one person to the next.
Since none of the five criteria, which define what is a vaccine, we cannot call this a vaccine.
So if this is not a vaccine, what is it?
Vaccines: The Basics
Vaccines contain the same germs that cause disease. (For example, measles vaccine contains measles virus, and Hib vaccine contains Hib bacteria.) But they have been either killed or weakened to the point that they don’t make you sick. Some vaccines contain only a part of the disease germ.
A vaccine stimulates your immune system to produce antibodies, exactly like it would if you were exposed to the disease. After getting vaccinated, you develop immunity to that disease, without having to get the disease first.
This is what makes vaccines such powerful medicine. Unlike most medicines, which treat or cure diseases, vaccines prevent them.
https://www.cdc.gov/vaccines/vpd/vpd-vac-basics.html
WHO: What is a vaccine
“Vaccines train your immune system to create antibodies, just as it does when it’s exposed to a disease. However, because vaccines contain only killed or weakened forms of germs like viruses or bacteria, they do not cause the disease or put you at risk of its complications.”
https://www.who.int/news-room/q-a-detail/vaccines-and-immunization-what-is-vaccination
Neither Moderna nor Pfizer claim that you develop immunity against COVID-19 nor that you can’t spread COVID-19 after receiving their COVID-19 “vaccines.”
In fact, in their clinical trials, they specify that they will not even test for immunity.
Unlike real vaccines, which use an antigen of the disease you’re trying to prevent, the COVID-19 injections do not include a reported Corona Virus in the injection people are getting. They contain synthetic RNA fragments encapsulated in a nanolipid carrier compound. The synthetic mRNA is NOT specific to the SARS-CoV2 virus and has been patented, because a natural mRNA cannot be patented.
The COVD-19 experimental injections do not actually impart immunity or inhibit transmissibility of the disease.
In other words, they are not designed to keep you from getting sick with SARS-CoV-2; they only are supposed to lessen your infection symptoms if or when you do get infected.
Therefore these products do not meet the legal or medical definition of a vaccine.
If vaccination is a public health measure that is supposed to protect and benefit the collective, then it would need to:
1. ensure that the individual who is vaccinated is rendered immune from the disease in question; and
2. that the vaccine inhibits transmission of the disease.
Only if these two outcomes can be scientifically proven can you say that vaccination protects and benefits the collective. But this is not the case with the mRNA experimental injections.
December 13, 2020
SARS-CoV-2 RNA reverse-transcribed and integrated into the human genome
https://www.biorxiv.org/content/10.1101/2020.12.12.422516v1
November 17, 2020
American Society of Gene + Cell Therapy
COVID-19 Vaccine Candidates Show Gene Therapy is a Viable Strategy
These findings, announced by Moderna on Nov. 16 and by Pfizer and its partner BioNTech on Nov. 9 (with an update on Nov. 18), demonstrate that gene therapy is a viable strategy for developing vaccines to combat COVID-19
https://www.asgct.org/research/news/november-2020/covid-19-moderna-nih-vaccine
Tal Zaks: Moderna chief medical officer Hacking the software of life
Tal Zaks, chief medical officer of Moderna, Inc., pharmaceutical manufacturer of the experimental mRNA technology injection. Zaks talks about how the mRNA naturally transmits critical information from the DNA our genes to the protein and says:
“THIS is THE critical information what the cell would actually do. Moderna thinks of it as an “Operation System”. If we can CHANGE this Software of Life, introducing or changing a line of code, that will have profund implications of everything. Imagine, instead the protein, we would give the instructions how to make the protein, so the body can make its own vaccine. We think of it as “Information Therapy”.”
This TEDx Beacon Street talk occurred in 2017.
Moderna CEO Describes the Vaccine As An Operating System
https://odysee.com/@unchained:9/moderna-ceo-vaccine-is-an-operating-system:c
Moderna – “Our Operating System”
Recognizing the broad potential of mRNA science, we set out to create an mRNA technology platform that functions very much like an operating system on a computer. It is designed so that it can plug and play interchangeably with different programs. In our case, the “program” or “app” is our mRNA drug – the unique mRNA sequence that codes for a protein.”
“Generally, the only thing that changes from one potential mRNA medicine to another is the coding region – the actual genetic code that instructs ribosomes to make protein. Utilizing these instruction sets gives our investigational mRNA medicines a software-like quality.”
https://www.modernatx.com/mrna-technology/mrna-platform-enabling-drug-discovery-development
mRNA COVID-19 vaccines do not prevent you from getting the infection, nor do they prevent its spread. They’re really experimental gene therapies.
This mysterious $2 billion biotech is revealing the secrets behind its new drugs and vaccines
“Moderna announced today that its experimental mRNA vaccine for COVID-19 achieved 94.5% protective efficacy in an interim analysis of a 30,000-person trial.”
https://www.sciencemag.org/news/2017/02/mysterious-2-billion-biotech-revealing-secrets-behind-its-new-drugs-and-vaccines
FDA Authorization
“CV19 Vaccine is an unapproved vaccine .. There is no FDA-approved vaccine to prevent CV19”
“FDA expects manufacturers to continue their clinical trials”
Emergency Use Authorization for Vaccines Explained
Page 2:
“…to support the issuance of an Emergency Use Authorization (EUA) under section …for an investigational vaccine to prevent COVID-19,….”
Page 4:
“It is FDA’s expectation that, following submission of an EUA request and issuance of an EUA, a sponsor would continue to collect placebo-controlled data in any ongoing trials for as long as feasible …. FDA’s recommendations regarding the safety and effectiveness data and information outlined below are essential to ensure that clinical development of a COVID-19 vaccine has progressed far enough that issuance of an EUA for the vaccine would not interfere with the ability of an ongoing Phase 3 trial to demonstrate effectiveness of the vaccine ..”
Page 11
CONSIDERATIONS FOR CONTINUING CLINICAL TRIALS FOLLOWING ISSUANCE OF AN EUA FOR A COVID-19 VACCINE FDA does not consider availability of a COVID-19 vaccine under EUA, in and of itself, as grounds for stopping blinded follow-up in an ongoing clinical trial. An EUA request should include strategies that will be implemented to ensure that ongoing clinical trials of the vaccine are able to assess long-term safety and efficacy (including evaluating for vaccine-associated ERD as well as decreased effectiveness as immunity wanes over time) in sufficient numbers of subjects to support vaccine licensure. …
https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
Emergency Use Authorization for Vaccines to Prevent COVID-19 Guidance for Industry, October 2020
Page 2:
“This guidance describes FDA’s current recommendations regarding the data and information needed to support the issuance of an Emergency Use Authorization (EUA) … for an investigational vaccine to prevent COVID-19…”
Page 4
“It is FDA’s expectation that, …a sponsor would continue to collect placebo-controlled data in any ongoing trials for as long as feasible … that issuance of an EUA for the vaccine would not interfere with the ability of an ongoing Phase 3 trial to demonstrate effectiveness of the vaccine to support licensure and …FDA notes that there would need to be an adequate plan for safety data collection among individuals vaccinated under an EUA.”
Page 11
“CONSIDERATIONS FOR CONTINUING CLINICAL TRIALS FOLLOWING ISSUANCE OF AN EUA FOR A COVID-19 VACCINE FDA does not consider availability of a COVID-19 vaccine under EUA, in and of itself, as grounds for stopping blinded follow-up in an ongoing clinical trial. An EUA request should include strategies that will be implemented to ensure that ongoing clinical trials of the vaccine are able to assess long-term safety and efficacy … in sufficient numbers of subjects to support vaccine licensure. These strategies should address how ongoing trial(s) will handle loss of follow-up information …
https://www.fda.gov/media/142749/download
December 23, 2020
Issuance of Authorization Letter to Pfizer
FDA – Pfizer COVID-19 EUA Letter of Authorization
Page 2:
“It is an investigational vaccine not licensed for any indication.”
“it is reasonable to believe that Pfizer-BioNTech COVID‑19 Vaccine may be effective.”
Page 4:
“Emergency Use Authorization”
“The Pfizer-BioNTech COVID‑19 Vaccine is authorized to be distributed, stored, further redistributed, and administered by emergency response stakeholders when packaged in the authorized manufacturer packaging (i.e., vials and cartons), despite the fact that the vial and carton labels may not contain information that otherwise would be required under the FD&C Act.”
Page 6:
“Pfizer Inc. will report to Vaccine Adverse Event Reporting System (VAERS):
• Vaccine administration errors whether or not associated with an adverse event;
• Serious adverse events (irrespective of attribution to vaccination);
• Cases of Multisystem Inflammatory Syndrome in children and adults; and
• Cases of COVID-19 that result in hospitalization or death, that are reported to
Pfizer Inc.
… Each periodic safety report is required to contain descriptive information which includes:
• A narrative summary and analysis of adverse events submitted during the reporting interval, including interval and cumulative counts by ..
• Newly identified safety concerns in the interval; and
• Actions taken since the last report because of adverse experiences …
Page 7:
“Pfizer Inc. will conduct post-authorization observational study(ies) to evaluate the association between Pfizer-BioNTech COVID-19 Vaccine and a pre-specified list of adverse events of special interest, along with deaths and hospitalizations, and severe COVID-19. … The study(ies) should be conducted in large scale databases with an active comparator. Pfizer Inc. will provide protocols and status update reports to the IND 19736 with agreed-upon study designs and milestone dates.”
Page 8:
“Vaccination providers administering Pfizer-BioNTech COVID‑19 Vaccine must report the following information associated with the administration of Pfizer-BioNTech COVID‑19 Vaccine of which they become aware to VAERS ..:
• Vaccine administration errors whether or not associated with an adverse event
• Serious adverse events (irrespective of attribution to vaccination)
• Cases of Multisystem Inflammatory Syndrome in children and adults
• Cases of COVID-19 that result in hospitalization or death
Complete and submit reports to VAERS ..
Page 9:
“All descriptive printed matter, advertising, and promotional material relating to the use of the Pfizer-BioNTech COVID‑19 Vaccine clearly and conspicuously shall state that:
• This product has not been approved or licensed by FDA, but has been authorized for emergency use by FDA”
https://www.fda.gov/media/144412/download
December 18, 2020
Issuance of Authorization Letter to Moderna
FDA – Moderna COVID-19 Vaccine EUA Letter of Authorization
Page 1:
“It is an investigational vaccine not licensed for any indication.”
“it is reasonable to believe that Moderna COVID‑19 Vaccine may be effective.”
Page 4:
“The Moderna COVID-19 Vaccine vial label and carton labels are clearly marked for “Emergency Use Authorization.” The Moderna COVID‑19 Vaccine is authorized to be distributed….despite the fact that the vial and carton labels may not contain information that otherwise would be required under the FD&C Act.”
Page 6:
“ModernaTX, Inc. will report to Vaccine Adverse Event Reporting System (VAERS):
• Vaccine administration errors whether or not associated with an adverse event;
• Serious adverse events (irrespective of attribution to vaccination);
• Cases of Multisystem Inflammatory Syndrome in adults; and
• Cases of COVID-19 that result in hospitalization or death, that are reported to ModernaTX, Inc.
These reports should be submitted to VAERS … Each periodic safety report is required to contain descriptive information which includes:
• A narrative summary and analysis of adverse events submitted during the reporting interval, including interval and cumulative counts by age groups, special populations (e.g., pregnant women), and adverse events of special interest.
• Newly identified safety concerns in the interval; and
• Actions taken since the last report because of adverse experiences …
Page 7:
“ModernaTX, Inc. will conduct post-authorization observational studies to evaluate the association between Moderna COVID-19 Vaccine and a pre-specified list of adverse events of special interest, along with deaths and hospitalizations, and severe COVID-19. … The studies should be conducted in large scale databases with an active comparator.
Page 8:
“Vaccination providers administering Moderna COVID‑19 Vaccine must report the following information associated with the administration of Moderna COVID‑19 Vaccine …:
• Vaccine administration errors whether or not associated with an adverse event
• Serious adverse events (irrespective of attribution to vaccination)
• Cases of Multisystem Inflammatory Syndrome in adults
• Cases of COVID-19 that result in hospitalization or death
Complete and submit reports to VAERS online ….
Page 9:
“descriptive printed matter, advertising, and promotional material relating to the use of the Moderna COVID‑19 Vaccine clearly and conspicuously shall state that:
• This product has not been approved or licensed by FDA,”
https://www.fda.gov/media/144636/download
Revised February 25, 2021
FACT SHEET FOR RECIPIENTS AND CAREGIVERS
EMERGENCY USE AUTHORIZATION (EUA) OF
THE PFIZER-BIONTECH COVID-19 VACCINE
“The Pfizer-BioNTech COVID-19 Vaccine is a vaccine and may prevent you from getting COVID-19.”
“The Pfizer-BioNTech COVID-19 Vaccine is an unapproved vaccine”
“There is no U.S. Food and Drug Administration (FDA) approved vaccine to prevent COVID-19.”
“The Pfizer-BioNTech COVID-19 Vaccine may not protect everyone.”
“There is no FDA-approved vaccine to prevent COVID-19.”
“The Pfizer-BioNTech COVID-19 Vaccine has not undergone the same type of review as an FDA-approved or cleared product.”
December 2020
FACT SHEET FOR RECIPIENTS AND CAREGIVERS EMERGENCY USE AUTHORIZATION (EUA) OF THE MODERNA COVID-19 VACCINE
Page 1:
“There is no U.S. Food and Drug Administration (FDA) approved vaccine to prevent COVID-19.”
“The Moderna COVID-19 Vaccine is an unapproved vaccine that may prevent COVID-19. There is no FDA-approved vaccine to prevent COVID-19.”
Page 2:
“The Moderna COVID-19 Vaccine is an unapproved vaccine”.
“In an ongoing clinical trial, the Moderna COVID-19 Vaccine has been…”
Page 3:
“The Moderna COVID-19 Vaccine is still being studied in clinical trials.”
Page 5:
“The Moderna COVID-19 Vaccine has not undergone the same type of review as an FDA- approved or cleared product”.
https://www.fda.gov/media/144638/download
December 1, 2020
BioNTech Press Release
Pfizer and BioNTech Receive Authorization in the European Union for COVID-19 Vaccine
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) announced today that the European Commission (EC) has granted a conditional marketing authorization (CMA) to Pfizer and BioNTech for COMIRNATY® (also known as BNT162b2), for active immunization to prevent COVID-19 caused by SARS-CoV-2 virus, in individuals 16 years of age and older.
The vaccine has now been granted a conditional marketing authorization, emergency use authorization or temporary authorization in more than 40 countries worldwide, including all 27 EU member states.
– Do not administer Pfizer-BioNTech COVID-19 Vaccine to individuals with known history of a severe allergic reaction
– The Pfizer-BioNTech COVID-19 Vaccine may not protect all vaccine recipients.
– Severe allergic reactions have been reported following the Pfizer-BioNTech COVID-19Vaccine during mass vaccination outside of clinical trials. Additional adverse reactions, some of which may be serious, may become apparent with more widespread use of the Pfizer-BioNTech COVID-19 Vaccine.
Available data on Pfizer-BioNTech COVID-19 Vaccine administered to pregnant women are insufficient
– Data are not available to assess the effects of Pfizer-BioNTech COVID-19 Vaccine on the breastfed infant or on milk production/excretion.
https://www.globenewswire.com/news-release/2020/12/21/2148959/0/en/Pfizer-and-BioNTech-Receive-Authorization-in-the-European-Union-for-COVID-19-Vaccine.html
November 30, 2020
Emergency Use Authorization (EUA) for an Unapproved Product
Review Memorandum
https://www.fda.gov/media/144673/download
https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine#additional
November 20, 2020
Emergency Use Authorization (EUA) for an Unapproved Product
Review Memorandum
https://www.fda.gov/media/144416/download
https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
November 20, 2020
Emergency Use Authorization for COVID-19 Vaccines Explained
Based on this declaration and determination, FDA may issue an EUA after FDA has determined that the following statutory requirements are met (section 564 of the FD&C Act (21 U.S.C. 360bbb-3)) (Ref. 3):
• The chemical, biological, radiological, or nuclear (CBRN) agent referred to in the March 27, 2020 EUA declaration by the Secretary of HHS (SARS-CoV-2) can cause a serious or life-threatening disease or condition.
• Based on the totality of scientific evidence available, including data from adequate and well-controlled trials, if available, it is reasonable to believe that the product may be effective to prevent, diagnose, or treat such serious or life-threatening disease or condition that can be caused by SARS-CoV-2.
• The known and potential benefits of the product, when used to diagnose, prevent, or treat the identified serious or life-threatening disease or condition, outweigh the known and potential risks of the product.
• There is no adequate, approved, and available alternative to the product for diagnosing, preventing, or treating the disease or condition.
https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
The COVID-19 vaccines should never have received the EUA approval because at least the last three requirements have never been met!
February 4, 2020
COVID-19 vaccine immunity from liability
The Declaration was effective as of February 4, 2020
The Secretary of the United States Department of Health & Human Services (HHS), Alex M. Azar III, has granted the companies selling and those involved in virtually any other activity related to any COVID-19 vaccine immunity from liability for any injuries caused by these products.
If the Declaration was already effective as of February 4, 2020 you have to wonder when they discussed, debated and wrote this declaration. They must have done so BEFORE we even knew about COVID-19 !
https://www.govinfo.gov/content/pkg/FR-2020-03-17/pdf/2020-05484.pdf
December 1, 2020
On December 1, 2020, the ex-Pfizer head of respiratory research Dr. Michael Yeadon and the lung specialist and former head of the public health department Dr. Wolfgang Wodarg filed an application with the EMA, the European Medicine Agency responsible for EU-wide drug approval, for the immediate suspension of all SARS CoV 2 vaccine studies, in particular the BioNtech/Pfizer study on BNT162b (EudraCT number 2020-002641-42).
“… this is called Antibody Dependent Enhancement (ADE), and is a common
problem with Dengue Virus, Ebola Virus, HIV, RSV, and the family of coronaviruses. In fact, this problem of ADE is a major reason why many previous vaccine trials for other coronaviruses failed. Major safety concerns were observed in animal models. If ADE occurs in an individual, their response to the virus can be worse than their response if they had never developed an antibody in the first place. This can cause a hyper-inflammatory response, a cytokine storm, and a generally dysregulation of the immune system that allows the virus to cause more damage to our lungs and other organs of our body. In addition, new cell types throughout our body are now susceptible to viral infection due to the additional viral entry pathway. There are many studies that demonstrate that ADE is a persistent problem with coronaviruses in general, and in particular, with SARS-related viruses. ADE has proven to be a serious challenge with coronavirus vaccines, and this is the primary reason many of such vaccines have failed in early in-vitro or animal trials. For example, rhesus macaques who were vaccinated with the Spike protein of the SARS-CoV virus demonstrated severe acute lung injury when challenged with SARS-CoV, while monkeys who were not vaccinated did not. Similarly, mice who were immunized with one of four different SARS-CoV vaccines showed histopathological changes in the lungs with eosinophil infiltration after being challenged with
There are some concerning issues with the trial designs, spelled out by Dr. Peter Doshi in the British Medical Journal. Dr. Doshi focuses on the two biggest issues. First, none of the leading vaccine candidate trials is designed to test if the vaccine can reduce severe COVID-19 symptoms, defined as: hospital admissions, ICU or death. And, second, the trials are not
designed to test if the vaccine can interrupt transmission.”
November 2020
Covid-19 Vaccine Clinical Trials:
Failure to Properly Assess Safety and Efficacy
ICAN sued the FDA in federal court and filed several petitions and a a Petition for a Stay of Action with the FDA which asks that the agency stay, or pause, any action related to the trials until the requested actions in the efficacy petition are implemented.
September 25, 2020
Robert F. Kennedy Jr Letter to FDA
“We are writing to you to request that the FDA and Center for Biological Evaluation and Research investigate Moderna’s mRNA vaccine that contains polyethylene glycol (PEG), a molecule which approximately 8% of the U.S. population has highly elevated levels of antibodies. Injecting a PEG-containing vaccine into individuals with pre-existing PEG antibodies could lead to life-threatening anaphylaxis.1 Children’s Health Defense has grave concerns that the Phase III clinical trial of Moderna’s mRNA-1273 SARS-CoV-2 vaccine may be non-compliant with the Protection of Human Subjects.”
https://childrenshealthdefense.org/wp-content/uploads/RFK_Jr_Letter_toFDA-_CBER-9-25-20.pdf
Informed Consent
According to the Nuremberg Code, as well as the Helsinki Declaration, a test as well as a medical treatment is only permitted if participants in such tests are extensively informed about the facts, and the possible risks of the vaccine or the medicine are explained to them in depth. They also must give their voluntary consent to this test or medical treatment if possible in writing.
However, millions of people who are currently being vaccinated are under the false assumption that the vaccines have already been approved and tested. They are therefore receiving the vaccinations under false premises. The infringement by those who carry out these vaccinations can be legally considered as serious bodily injury in each and every case.
The same applies to black-mailing by announcing that you cannot fly without having been vaccinated, that immunization passes may be required, that you need this vaccination to keep your job, etc…. Each of these demands are illegal and violate the internationally recognized medical and scientific ethics and human rights established by the Nuremberg Code, as well as the Helsinki Declaration.
It should be noted that mandatory vaccination would be in direct violation of The Nuremberg Code, a violation of the World Medical Association
Declaration of Helsinki and a violation of article 6 of the UNESCO 2005 Universal Declaration On Bioethics And Human Rights.
1947
The Nuremberg Code
http://www.cirp.org/library/ethics/nuremberg/
1964
World Medical Association
Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects
https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
2005
UNESCO – Universal Declaration on Bioethics and Human Rights
http://portal.unesco.org/en/ev.php-URL_ID=31058&URL_DO=DO_TOPIC&URL_SECTION=201.html
Covid vaccines can not be administered without informed consent & the ability to exercise free power of choice by the recipient because nobody can provide true and in-depth information about the experimental vaccines and their adverse effects and longterm consequences. Therefore any forcing, coaxing, coercing, persuading, seducing, blackmailing and also the act of administering is in violation of the following medical laws and standards:
🛡 Hippocrates oath (-460 // 377): ′′ I will not give anyone poison, if asked, nor take the initiative of such a suggestion. ′′
🛡 Code of Medical Conduct, Article 36:
Article R4127- 36 of the Public Health Code: ′′ The consent of the person examined or treated must be sought in all cases. When the patient, in a state of expressing his will, refuses the investigation or treatment proposed, the doctor must respect this refusal after informing the patient of his consequences ′′
🛡 Nuremberg Code (1947): ′′ The consent of the human subject is absolutely essential. The International Covenant on Civil and Political Rights resumed this ban against unintentional experimentation, in its 1966 text, which states: no one may be subjected without his consent to medical or scientific experiment
🛡 Geneva statement for doctors (1948): ′′ I will respect the autonomy and dignity of my patient. I will not use my medical knowledge to infringe human rights and civil liberties, even under force. I will keep absolute respect for human life, from conception. I will consider my patient’s health as my first concern ′′
🛡 Helsinki Declaration (1996) signed by 45 countries including France:
Article 25: ′′ The participation of persons capable of giving informed consent to medical research must be a voluntary act. No person capable of giving their informed consent can be involved in a search without giving their free and informed consent ′′
🛡 Oviedo Convention (1997) signed by 29 countries including France):
Article 5: ′′ An intervention in the health field may only be carried out after the data subject has given free and informed consent. This person is given prior adequate information about the purpose and nature of the intervention, as well as its consequences and risks. The data subject may, at any time, freely withdraw his consent ′′
🛡 Loi Kouchner (March 4, 2002):
Article 111-4: ′′ Every person shall make decisions concerning his health with the healthcare professional and taking into account the information he provides him / her. The doctor must respect the will of the person after informing them of the consequences of their choices. If the person’s willingness to refuse or discontinue treatment puts his or her life at risk, the doctor must do everything to convince him or her to accept the much needed care. No medical or treatment can be practiced without the free and informed consent of the person and this consent can be withdrawn at any time
🛡 Salvetti stop (2002): No medical treatment is mandatory in the European Union: ′′ As a non-voluntary medical treatment, mandatory vaccination is an interference with the right to privacy, guaranteed by Article 8 of the European Convention on Human Rights and Fundamental Freedoms ′′ (Salvetti v Italy-ECHR decision of 9 July 2002; No. 42197/98)
🛡 Universal Declaration on Bioethics and Human Rights (19 October 2005)
UNESO
Article 6 – Consent
1. Any preventive, diagnostic and therapeutic medical intervention is only to be carried out with the prior, free and informed consent of the person concerned, based on adequate information. The consent should, where appropriate, be express and may be withdrawn by the person concerned at any time and for any reason without disadvantage or prejudice.
… In no case should a collective community agreement or the consent of a community leader or other authority substitute for an individual’s informed consent.
🛡 French Civil Code:
Article 16-1: ′′ Everyone has the right to respect their own bodies. The body is inviolable ′′
🛡 Council of Europe resolution 2361 (28 January 2021): advisory opinion: the Assembly urges member states and the European Union:
Article 731: ′′ To ensure that citizens are informed that vaccination is not mandatory and that no one is under political, social or other pressure to get vaccinated, if he or she does not wish to do so personally ′′
Article 732: ′′ To ensure that no one is discriminated against for not being vaccinated, because of potential health risk or for not wanting to get vaccinated
October 28, 2020
Informed consent disclosure to vaccine trial subjects of risk of COVID‐19 vaccines worsening clinical disease
Informed consent disclosure to vaccine trial subjects of risk of COVID‐19 vaccines worsening clinical disease
“Given the strong evidence that ADE is a non‐theoretical and compelling risk for COVID‐19 vaccines and the “laundry list” nature of informed consents, disclosure of the specific risk of worsened COVID‐19 disease from vaccination calls for a specific, separate, informed consent form and demonstration of patient comprehension in order to meet medical ethics standards. The informed consent process for ongoing COVID‐19 vaccine trials does not appear to meet this standard. While the COVID‐19 global health emergency justifies accelerated vaccine trials of candidates with known liabilities, such an acceleration is not inconsistent with additional attention paid to heightened informed consent procedures specific to COVID‐19 vaccine risks.”
“The specific and significant COVID‐19 risk of ADE should have been and should be prominently and independently disclosed to research subjects currently in vaccine trials, as well as those being recruited for the trials and future patients after vaccine approval, in order to meet the medical ethics standard of patient comprehension for informed consent.”
https://onlinelibrary.wiley.com/doi/10.1111/ijcp.13795
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7645850/
Federal law prohibits employers and others from requiring vaccination with a Covid-19 vaccine distributed under an EUA
https://www.statnews.com/2021/02/23/federal-law-prohibits-employers-and-others-from-requiring-vaccination-with-a-covid-19-vaccine-distributed-under-an-eua/
A Harvard Vaccine Injury Study conducted from 2007 to 2010, reveals on page 6 a 1 % report rate.
The Department of Health and Human Services (HHS) gave Harvard Medical School a $1 million dollar grant to track VAERS reporting at Harvard Pilgrim Healthcare for 3 years and to create an automated reporting system which would revolutionize the VAERS reporting system- transforming it from “passive” to “active.”
“Adverse events from drugs and vaccines are common, but underreported. […] Likewise, fewer than 1% of vaccine adverse events are reported. Low reporting rates preclude or slow the identification of ‘problem’ drugs and vaccines that endanger public health. New surveillance methods for drug and vaccine adverse effects are needed.”
More information at: Harvard Study
Adverse Events
Check the latest numbers from VAERS and EudraVigilance on our Adverse Events Page -> here.
2/23/2021
In the USA the CDC provides VAERS, a well established PASSIVE Vaccine Adverse Event Reporting System.
On 2/23/21 the system listed the following numbers for the COVID-19 vaccines:
799 deaths
610 life threatening events
1,851 hospitalized events
20,609 adverse events
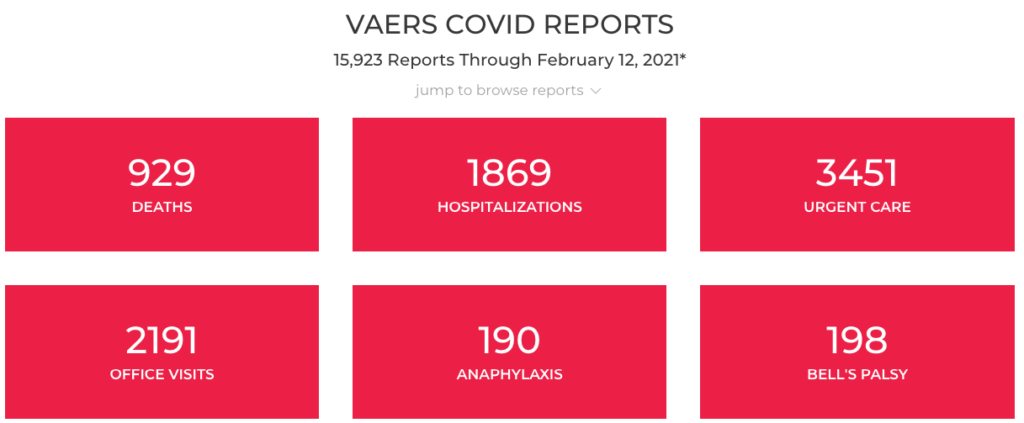
on 2/23/2021 OpenVAERS, an online platform collecting the data directly from the VAERS system displayed
929 deaths
1869 hospitalizations
3451 Urgent Care
2191 Office Visits
https://vaers.hhs.gov/
https://wonder.cdc.gov/vaers.html
https://www.openvaers.com/covid-data
2/18/2021
Selected Adverse Events Reported after COVID-19 Vaccination
“Over 52 million doses of COVID-19 vaccines were administered in the United States from December 14, 2020, through February 14, 2021. During this time, VAERS received 934 reports of death among people who received a COVID-19 vaccine.”
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html#:~:text=Over%2052%20million%20doses%20of,received%20a%20COVID-19%20vaccine
V-safe after vaccination health checker
There is also the V-safe after vaccination health checker
V-safe is a smartphone-based tool that uses text messaging and web surveys to provide personalized health check-ins after someone received a COVID-19 vaccine. It is not possible for the public to access the data from this data base.
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html
It is not clear if data collected on V-safe is also provided to VAERS. Since VAERS is passive & V-safe is not necessarily feeding into VAERS how can we know the correct numbers?
What are the actual numbers and who is listing/reporting them when they are not all in one single official system?
Electronic Support for Public Health–Vaccine Adverse
Event Reporting System (ESP:VAERS)
Adverse events from drugs and vaccines are common, but underreported. Although 25% of
ambulatory patients experience an adverse drug event, less than 0.3% of all adverse drug events
and 1-13% of serious events are reported to the Food and Drug Administration (FDA).
Likewise, fewer than 1% of vaccine adverse events are reported.
https://digital.ahrq.gov/sites/default/files/docs/publication/r18hs017045-lazarus-final-report-2011.pdf
March 2, 2021
Germany
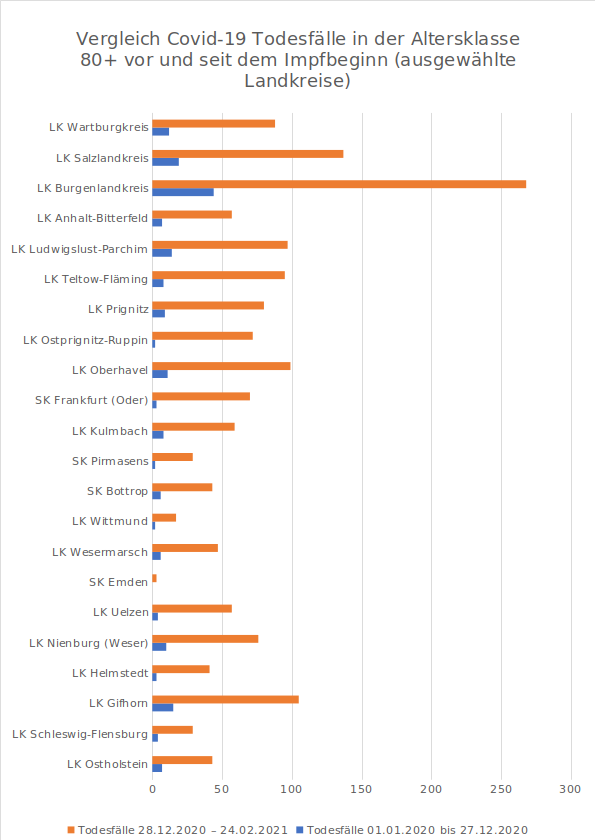
The number of people who died in the past two months is at least as high as it was during the previous 12 months in almost every county. In 51 counties, mortality is more than four times higher, and in 22 of them, it is more than six times higher.
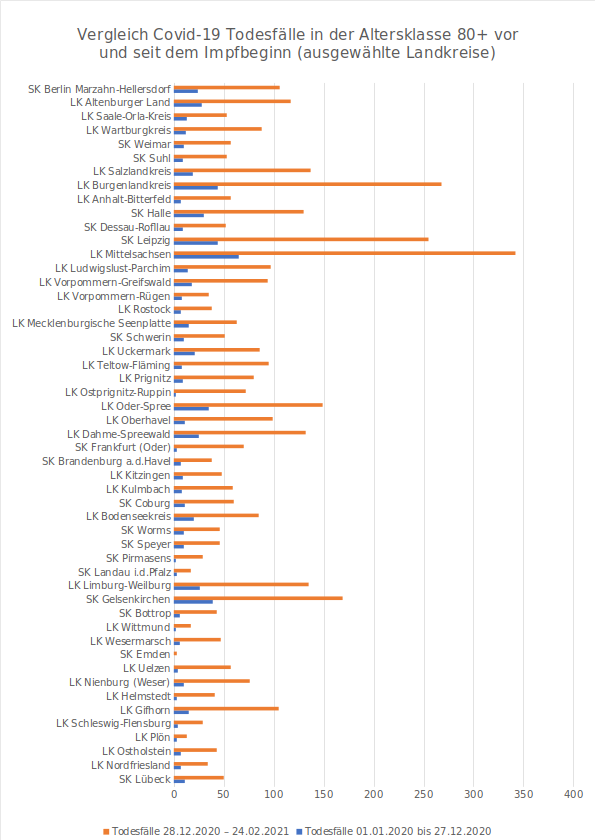
The numbers have been checked by Martin Adam using the data base of the official Robert-Koch-Institute/RKI in Germany (Germany’s CDC). Everybody can access the database here „Application Programming Interface (API)“. Unfortunately getting and displaying this data requires a great deal of technical knowledge and the hurdle is quite difficult. Martin Adam has written a program with which he retrieves the data from the RKI and performed a special analysis of the data for people in the age group 80 and older.
Please find the charts below.
The article is in German.
https://corona-blog.net/2021/03/02/dramatischer-anstieg-der-todesfaelle-unter-senioren-seit-beginn-der-corona-schutzimpfungen/
22 counties where mortality is over six times higher in less than 2 months than in (almost) all of 2020
Comparison COVID-19 Deaths in the age group 80+ before and since vaccination began (selected counties)

51 counties with more than four times the mortality rate
Comparison COVID-19 Deaths in the age group 80+ before and since vaccination began (selected counties)

Adverse Effects Checks
If you are vaccinated in the trial and don’t drop out, they only follow you for adverse effects for the following periods:
Pfizer/BionTech:
1 month after second does
6 months for serious adverse events
Moderna :
8/7 days after first dose
36/7 days after second does up to 57 days
AstraZeneca/Oxford:
1 month after second does
6 months for serious adverse events
But FDA doesn’t consider certain side effects serious, so they will only track one month. Including but not limited to alopecia, autoimmune disease, lupus erythematous, vasculitis, Bell’s Palsy, hypotonia, migraine, myelitis, neuropathy, seizures, mental disorders, rhinitis, and vertigo. Most of these take months or years to show up, when the time frame to track has long ended.
The vaccines are still in the experimental trial phase until 2023 / 2022
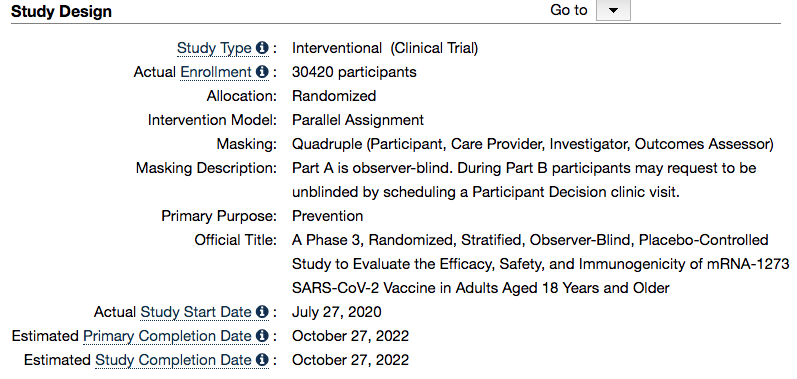
Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals
Pfizer – Estimated Study Completion Date: January31, 2023
https://www.clinicaltrials.gov/ct2/show/NCT04368728
A Study to Evaluate the Safety, Reactogenicity, and Effectiveness of mRNA-1273 Vaccine in Adolescents 12 to <18 Years Old to Prevent COVID-19
Moderna – Estimated Study Completion Date: October 27, 2022
https://clinicaltrials.gov/ct2/show/NCT04470427
Research Studies
December 28, 2020
COVID-19 mRNA Vaccines
“Researchers have been trying to develop a coronavirus vaccine since the Severe Acute Respiratory Syndrome (SARS-1) outbreak in 2002. Thus, over a span of 18 years there have been numerous coronavirus vaccine animal studies conducted, which unfortunately demonstrated significant and serious side-effects. Either the animals were not completely protected, became severely ill with accelerated autoimmune conditions, or died.”
“Generally, the conclusion of some of those studies was that great caution needs to be exercised when moving forward to human trials primarily because of the potential of accelerated autoimmunity reaction.”
“After two decades of failed animal trials, the question is posed as to why fast-tracking coronavirus vaccine will now result in a different outcome? Given that many of these fast-track trials have bypassed animal studies, are only performed on healthy volunteers and children (not the elderly or those with pre-morbidities), and that trials are conducted without an inert double-blind placebo-controlled environment, and are not given sufficient time to observe effects on the human trials, there is a serious safety concern. Many, many virologists, and epidemiologists feel this fast-track policy is a recipe for mass disaster.”
https://www.biologicalmedicineinstitute.com/post/covid-19-mrna-vaccines
December 17, 2020
Vaccines and Related Biological Products Advisory Committee Meeting
Moderna / For the primary efficacy endpoint, the case definition for a confirmed COVID-19 case was defined as: At least TWO of the following systemic symptoms: Fever (≥38ºC), chills, myalgia, headache, sore throat, new olfactory and taste disorder(s), OR At least ONE of the following respiratory signs/ symptoms: cough, shortness of breath or difficulty breathing, OR clinical or radiographical evidence of pneumonia; and NP swab, nasal swab, or saliva sample (or respiratory sample, if hospitalized) positive for SARS-CoV-2 by RT-PCR.
“FDA has not independently verified the complete safety dataset and analyses”
“It is not possible to assess sustained efficacy over a period longer than 2 months.”
“The study was not designed to assess the benefit in individuals with prior SARS-CoV-2 infection.”
“Data are limited to assess the effect of the vaccine in preventing asymptomatic infection as measured by detection of the virus and/or detection of antibodies against non-vaccine antigens that would indicate infection rather than an immune response induced by the vaccine. Additional evaluations will be needed to assess the effect of the vaccine in preventing asymptomatic infection”
“Data are limited to assess the effect of the vaccine against transmission of SARS-CoV-2 from individuals who are infected despite vaccination.”
“Additional evaluations including data from clinical trials and from vaccine use post-authorization will be needed to assess the effect of the vaccine in preventing virus shedding and transmission, in particular in individuals with asymptomatic infection.”
“Following authorization of the vaccine, use in large numbers of individuals may reveal additional, potentially less frequent and/or more serious adverse events”
December 10.2020
Vaccines and Related Biological Products Advisory Committee Meeting
Pfizer / For the primary efficacy endpoint, the case definition for a confirmed COVID-19 case was the presence of at least one of the following symptoms and a positive SARS-CoV-2 NAAT within 4 days of the symptomatic period:
Fever
New or increased cough
New or increased shortness of breath
Chills
New or increased muscle pain
New loss of taste or smell
Sore throat
Diarrhea
Vomiting
“Pregnant women were excluded or discontinued from study vaccination. Unsolicited adverse effects related to pregnancy included spontaneous abortion and retained products of conception. Pregnancy outcomes are otherwise unknown at this time.”
“It is not possible to assess sustained efficacy over a period longer than 2 months.”
“A larger number of individuals at high risk of COVID-19 and higher attack rates would be needed to confirm efficacy of the vaccine against mortality.”
“Data are limited to assess the effect of the vaccine against transmission of SARS-CoV-2 from individuals who are infected despite vaccination.”
“Additional evaluations including data from clinical trials and from vaccine use post-authorization will be needed to assess the effect of the vaccine in preventing virus shedding and transmission, in particular in individuals with asymptomatic infection.”
December 9, 2020
UK issues anaphylaxis warning on Pfizer vaccine after adverse reactions
“Britain’s medicine regulator said anyone with a history of anaphylaxis to a medicine or food should not get the Pfizer-BioNTech COVID-19 vaccine” MHRA Chief Executive June Raine said in a statement.
“The allergic reactions may have been caused by a component of Pfizer’s vaccine called polyethylene glycol, or PEG, which helps stabilize the shot and is not in other types of vaccines.”
https://www.reuters.com/article/idUSKBN28J1D1
October 21, 2020
Will covid-19 vaccines save lives? Current trials aren’t designed to tell us.
“Ideally, you want an antiviral vaccine to do two things . . .
1. reduce the likelihood you will get
severely ill and go to the hospital, and
2. prevent infection and therefore interrupt disease
transmission.
Yet the current phase III trials are not actually set up
to prove either”.
The trials are not designed to demonstrate a reduction in transmission, due to “operational realities”.
Peter Doshi, assistant professor of pharmaceutical health services research at the University of Maryland School of Pharmacy and associate editor at The BMJ.
https://www.bmj.com/content/371/bmj.m4037
September 9, 2020
Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies
“Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies “ADE has been observed in SARS, MERS and other human respiratory virus infections including RSV and measles, which suggests a real risk of ADE for SARS-CoV-2 vaccines and antibody-based interventions.
Going forwards, it will be crucial to evaluate animal and clinical datasets for signs of ADE, and to balance ADE-related safety risks against intervention efficacy if clinical ADE is observed. Ongoing animal and human clinical studies will provide important insights into the mechanisms of ADE in COVID-19. Such evidence is sorely needed to ensure product safety in the large-scale medical interventions that are likely required to reduce the global burden of COVID-19.”
https://www.nature.com/articles/s41564-020-00789-5.pdf
Powered By EmbedPress
May 12, 2020
Fauci tells Congress: ‘There’s no guarantee that the vaccine is actually going to be effective’
Another worry among epidemiologists, Fauci said, is that the vaccine backfires and strengthens the virus.
There have been at least two vaccines in the past that have produced a “suboptimal response,” he said. “And when the person gets exposed, they actually have an enhanced pathogenesis of the disease, which is always worrisome. So we want to make sure that that doesn’t happen. Those are the two major unknowns.”
April 24, 2019
mRNA per se cannot alter the DNA. However, there is a mechanism, used by retroviruses, where mRNA is used as a matrix to synthesize DNA which in turn can be incorporated into the genome (thus “altering” the host DNA). This will require only two viral enzymes: reverse transcriptase and invertase.
So, in theory, for those who have a retroviral infection such as HIV or human T-lymphotropic virus (HTLV), there is a chance that this could happen.
Also human genome contains up to 8% of retrovirus-like sequences whose function is not very well understood, and while some of these HERVs sequences seem to be defective, others may be capable of producing functional proteins and thus provide tools for the integration.
I recommend this open access scientific review paper comparing DNA and mRNA vacs technologies from April 2019. It also talks about some lovely side effects and such.
https://pubmed.ncbi.nlm.nih.gov/31022829/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6631684/
April 2012
Immunization with SARS Coronavirus Vaccines Leads to Pulmonary Immunopathology on Challenge with the SARS Virus
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3335060/pdf/pone.0035421.pdf
UK government guidelines for Pfizer vaccine:
– No reproductive toxicity studies completed
– Avoid pregnancy for > 2 months
– Risk to the newborns cannot be excluded
– Should not be used during breast-feeding
– Unknown if it has an impact on fertility
– Animal studies into potential toxicity to reproduction and development have not been completed.
December 19, 2020
Anaphylaxis Following m-RNA COVID-19 Vaccine Receipt
The chart below from the CDC’s own website indicates as of December 18th, 112,807 Americans have been vaccinated with 3,150 “health impacts.” That’s about 3 %. The CDC chart defines “health impacts” as “unable to perform normal daily activities, unable to work, required care from doctor or health care professional.”
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/slides-12-19/05-COVID-CLARK.pdf
December 9, 2020
Allergic reactions associated with Covid-19 vaccine, BNT162b2
https://www.pharmaceutical-technology.com/features/covid19-vaccine-allergic-reactions/
November 2020
BIONTECH
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a‑16 OR 15d‑16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
FOR THE MONTH OF NOVEMBER 2020
BionTech:
– even if we demonstrate a sufficient safety profile for BNT162 we may not be able to demonstrate sufficient efficacy in subsequent trials to obtain regulatory approval.
– BNT162b2 Phase 2b/3 trial is ongoing until 2023
– Future results in clinical trials of BNT162 may not be as positive when compared to the antibody levels in other samples of convalescent sera.
– indications of immunogenicity and the duration of immunity observed in our Phase 1/2 trials may not be predictive of the achievement of clinically relevant endpoints.
– In addition, by definition our Phase 1/2 clinical trials are designed to evaluate only safety and not efficacy.
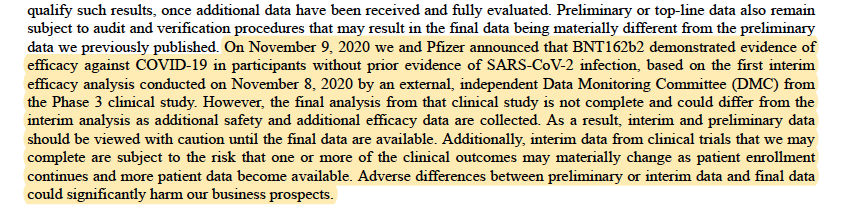
– On November 9, 2020 we and Pfizer announced that BNT162b2 demonstrated evidence of efficacy against COVID-19 in participants without prior evidence of SARS-CoV-2 infection, based on the first interim efficacy analysis conducted on November 8, 2020 by an external, independent Data Monitoring Committee (DMC) from the Phase 3 clinical study. However, the final analysis from that clinical study is not complete and could differ from the interim analysis as additional safety and additional efficacy data are collected. Failure to adequately demonstrate safety or to eventually demonstrate sufficient efficacy of BNT162 could delay or prevent us from receiving regulatory approval of BNT162 and there can be no assurance that BNT162 will be approved in a timely manner, if at all.
– We may be unable to produce a successful COVID-19 vaccine and establish a competitive market share for our vaccine before a competitor or before the COVID-19 outbreak is effectively contained or the risk of coronavirus infection is significantly diminished.
– Our product candidates may not work as intended, may cause undesirable side effects or may have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval
– Results of our trials could reveal a high and unacceptable severity and prevalence of side effects.
– If unacceptable side effects arise in the development of our product candidates, we, the FDA, competent authorities of European Union member states, ethics committees, the institutional review boards, or IRBs, at the institutions in which our studies are conducted, or the Data Safety Monitoring Board, or DSMB, could suspend or terminate our clinical trials.
– Medicines used at centers to help manage adverse side effects of our product candidates may not adequately control the side effects and may have a detrimental impact on the efficacy of the treatment.
– any clinical trials may not be sufficient to determine the effect and safety consequences of taking our product candidates over a multi-year period.
– Clinical testing is expensive and complex and can take many years to complete. Its outcome is inherently uncertain.
– On November 9, 2020 we and Pfizer announced that BNT162b2 demonstrated evidence of efficacy against COVID-19 in participants without prior evidence of SARS-CoV-2 infection, based on the first interim efficacy analysis conducted on November 8, 2020 by an external, independent Data Monitoring Committee (DMC) from the Phase 3 clinical study. However, the final analysis from that clinical study is not complete and could differ from the interim analysis as additional safety and additional efficacy data are collected. As a result, interim and preliminary data should be viewed with caution until the final data are available. Additionally, interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Adverse differences between preliminary or interim data and final data could significantly harm our business prospects. https://investors.biontech.de/node/8746/html
Should I get a COVID-19 Vaccine?
https://www.icandecide.org/wp-content/uploads/2020/10/Flyer-Should-I-Get-a-Covid-19-Vaccine-1.pdf
They are paying $740 for people taking part in a COVID-19 vaccine study in the USA
Palantir has been developing software that federal health officials would use to manage the various vaccine data and identify any issues that could prevent Americans from getting the shots
https://www.wsj.com/articles/palantir-to-help-u-s-track-covid-19-vaccines-11603367276
We urgently need your support !!
There are many easy ways how to do this. Please find the information here:
>How to Support Us <
>Shop at our Affiliate Partner Shops<
Find books, nutritions, vitamins, groceries, outdoor products, travel deals, and much more on our pages:
Dietary Supplements, Immune Support
or please Donate here 🙂
A great explanation about COVID-19 vaccination
This animated video, “COVID Vaccine Secrets,” walks viewers through a long list of questions about the COVID vaccines, including many for which we may never have answers.
Here are the sources, please check them for yourself! Sources
March 16, 2021
Prof. Sucharit Bhakdi:
The Time to Act is NOW!
Statement on urgent Open Letter from Doctors and Scientists to EMA
Video
A great summary about COVID-19 vaccination
Most of the links in this article are from medical journals, the FDA, CDC, and other official entities that generally support vaccination.
Physician: Informed Consent For COVID Vaccine Requires Full Disclosure Of Risk & Liability, And Here It Is…
https://www.technocracy.news/physician-informed-consent-for-covid-vaccine-requires-full-disclosure-of-risk-liability-and-here-it-is/?print=pdf
March 12, 2021
Dr. Phillips on Vaccine Safety & Efficacy!
Video
February 7, 2021
Irish Professor Dolores Cahill and French Geneticist Alexandra H. Cause on Covid-19 Vaccine
Video
February 6, 2021
Dr. Vanessa Schmidt-Krüger über Risiken des mRNA Impfstoffs (German)
mRNA Impfstoff
January 23, 2021
Return to Wuhan: What Life Is Like One Year Later / NBC Nightly News
Dr. Wu Zunyou / Chinese Center For Disease Control
“They didn’t isolate the virus. That’s the issue.”
Why people will start dying a few months after the first mRNA vaccination
– Professor Dolores CaHill / Molecular Biologist and Immunologist is a world leading scientist and academic. She is best recognized as one of the world-renowned scientists in the field of Bio-Medicine. PhD in Immunology from Dublin City University in 1994. She was group leader of the Protein Technology Group in the Max-Planck-Institute of Molecular Genetics, Berlin, and is Professor of Translational Science at the UCD School of Medicine and Medical Sciences. Dolores has been a member of the Advisory Science Council to the Irish government and a member of the International Science Advisory Board.
– Dr. Alexandra Henrion-Claude / French biomedical researcher. Achievements include patents for bioinformatic tool in microRNA. She is one of the top experts of RNA in France and a geneticist. For years, she was a researcher at the French government’s Institut National de la Santé et de la Recherche Médicale (Inserm). She received many scientific awards in her career and has done work on epigenetics.
Dr. Larry Palevsky — Covid-19 Vaccine Safety Concerns
“I am afraid, based on the lack of knowledge that we have and the developing knowledge that we have, that the recovery rate from this vaccine won’t be nearly as high as the recovery rate from the illness itself.”
— Larry Palevsky, M.D. / Pediatrician at North Wellness Centre
October 22, 2020
Dr. James Lyons-Weiler
Dr. James Lyons-Weiler is the president and CEO of The Institute for Pure and Applied Knowledge and a research scientist with a PhD in Ecology, Evolution and Conservation in Biology, and a postdoctoral in Computational Molecular Biology from Penn State University.
COVID Vaccine on Trial: If You Only Knew . . .
World-renowned experts, medical doctors, lawyers, scientists talking about the COVID-19 vaccines.
https://mamm.org/covid-vaccine-on-trial-3/
BioNTech/Pfizer Impfstoff – Vorstellung der klinischen Studie 1 (German)
Dr. Vanessa Schmidt-Krüger / Scientist / Zellbiologin
Molecular Cardiovascular Research, Max-Delbrück-Centrum für Molekulare Medizin (MDC) Berlin, Germany
Dr. Vanessa Schmidt-Krueger
Zellbiologin Dr. Vanessa Schmidt Krüger über die Gefahren der mRNA-Impfung
German
Welche Gefahren gehen von den Lipid-Nanopartikeln aus ? BioNTech-Impfstoff
Dr. Vanessa Schmidt-Krueger
Zellbiologin Dr. Vanessa Schmidt Krüger über die Gefahren der mRNA-Impfung
German
https://www.corona-aufklaerung.tv/2021/02/16/zellbiologin-dr-vanessa-schmidt-krueger-ueber-die-gefahren-der-mrna-impfung/
English Transcript:
http://enformtk.u-aizu.ac.jp/howard/gcep_dr_vanessa_schmidt_krueger/
25% death rate after vaccination
8 deaths at care home after use of mRNA vaccine
Whistleblower interview by lawyers Reiner Füllmich and Viviane Fischer / Corona-Ausschuss
(German with English subtitles + whistleblower video)
https://lbry.tv/@shortXXvids:e/Berlin-Care-Home-Vaccine-Deaths—Whistleblower-report:f
Dr. Steve Hotze details exactly what a mRNA Non-vaccine actually is and what it can do to you!
https://rumble.com/veos2r-all-about-mrna-so-called-vaccines.html
All information is deemed accurate but not guaranteed and should be independently verified.