The PCR Test
We are in a PCR false positive pseudo-epidemic created by a scientifically meaningless PCR Test.
In mid-January 2020 a PCR (RT-PCR)diagnostic test protocol (“Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR” )was created …… find more information here. 👉🏼
The PCR test protocol (polymerase chain reaction) has been used to run hundreds of millions of PCR tests across the world since last spring in 2020. Its positive test results have been the basis for all kind of restrictions all around the world ever since.
Mass testing the healthy population with a PCR test which creates many false positives generates a “PCR false positive pseudo-epidemic”.
Please find more information about the test on this page. 👉🏼
Below you will find scientific, detailed explanations and information about the PCR test protocol (polymerase chain reaction) and why it generates false positives in in-depth studies and research papers published by scientists and experts below and on the right hand side.
July 21, 2021
On July 21, 2021 the CDC published the following “Lab Alert”:
Changes to the CDC RT-PCR for SARS-CoV-2 Testing
“After December 31, 2021, CDC will withdraw the request to the U.S. Food and Drug Administration (FDA) for Emergency Use Authorization (EUA) of the CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel, the assay first introduced in February 2020 for detection of SARS-CoV-2 only. CDC is providing this advance notice for clinical laboratories to have adequate time to select and implement one of the many FDA-authorized alternatives.”
…
“In preparation for this change, CDC recommends clinical laboratories and testing sites that have been using the CDC 2019-nCoV RT-PCR assay select and begin their transition to another FDA-authorized COVID-19 test. CDC encourages laboratories to consider adoption of a multiplexed method that can facilitate detection and differentiation of SARS-CoV-2 and influenza viruses. Such assays can facilitate continued testing for both influenza and SARS-CoV-2 and can save both time and resources as we head into influenza season.”
…
CDC / Lab Alert / 7/21/2021
So-
1. the EUA for the CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel will end on December 31, 2021
2. FDA authorized COVID-19 types of SARS-CoV-2 and COVID-19 related IVDs / In Vitro Diagnostics EUAs
3. The CDC actually admits that the PCR tests never could differentiate between the flu and SARS-C0V-2 !
July 15, 20231
Removal Lists of Tests That Should No Longer Be Used and/or Distributed for COVID-19
Long list of tests that should no longer be used and/or distributed for COVID-19.
FDA
July 27, 2021
June 1, 2021
This is an example of one of the manufacturers that uses “NASAL SWABS” out of more than 300 manufacturers. Interestingly it causes injuries or death.
The FDA has identified this recall as a Class I recall, the most serious type of recall.
Stop Using Innova Medical Group SARS-CoV-2 Antigen Rapid Qualitative Test
June 1, 2021
The performance of the SARS-CoV-2 RT-PCR test as a tool for detecting SARS-CoV-2 infection in the population
“In light of our findings that more than half of individuals with positive PCR test results are unlikely to have been infectious, RT-PCR test positivity should not be taken as an accurate measure of infectious SARS-CoV-2 incidence. Our results confirm the findings of others that the routine use of “positive” RT-PCR test results as the gold standard for assessing and controlling infectiousness fails to reflect the fact “that 50-75% of the time an individual is PCR positive, they are likely to be post-infectious”
NCBI
April 5, 2021
A brief report: Cerebrospinal fluid rhinorrhea after repetitive nasal swab testing for coronavirus disease 2019(COVID-19)
“Even though nasal swab sampling has become a reliable source of a diagnostic tool during COVID-19 pandemic, this case of CSF leak shows that nasopharyngeal swab testing can lead to iatrogenic outcomes due to its invasiveness.”
February 23, 2021
Positive results from UK single gene testing for SARS-COV-2 may be inconclusive, negative or detecting past infections
“Without diagnostic validation of the single gene call, for both the original and the B1.1.7 variant it can only be assumed that, in the absence of confirmatory testing, many of the reported positive results may in fact be inconclusive, negative or past infection for SARS-COV-2.”
“The proportion of positives called on single genes increased over time, suggesting a shift in testing policy around mid-November 2020 coincident with the reported significant increase in transmission of the new variant B1.1.7.”
“Unless the UK lighthouse laboratories have performed diagnostic validation of their single gene call, for both the original and the B1.1.7 variant, and there is no evidence of this in the public domain, it can only be assumed that, in the absence of confirmatory testing, many of the reported positive results may in fact be inconclusive, negative or from people who suffered past infection for SARS-COV-2. And even with diagnostic validation of the single gene call the UK lighthouse laboratories appear to be in breach of both the WHO emergency use assessment and, also to have potentially violated the ThermoFisher TaqPath kit instructions for use.”
https://arxiv.org/pdf/2102.11612v1.pdf
January 20, 2021
WHO changes PCR Test criteria calling into question the ability of PCR Test to detect COVID-19
In an updated guidance published on Jan. 20, 2021 the WHO said that lab experts and health care practitioners should also consider the patient’s history and epidemiological risk factors alongside the PCR test in diagnosing the coronavirus.
The World Health Organization published a memorandum on January 13, 2021 stating that a single positive PCR test should not be used for diagnosing Sars-Cov-2 infection.
WHO finally says:
Most PCR assays are indicated as an aid for diagnosis, therefore, health care providers must consider any result in combination with timing of sampling, specimen type, assay specifics, clinical observations, patient history, confirmed status of any contacts, and epidemiological information.
…… and with this clarifies that it is not a diagnostic test.
It never was, but WHO finally admits this openly.
The new guidance could result in significantly fewer daily cases, the false positive cases that created an environment of panic and fear which experts have been warning since last year.
WHO Information Notice for IVD Users 2020/05
Nucleic acid testing (NAT) technologies that use polymerase chain reaction (PCR) for detection of SARS-CoV-2
https://www.who.int/news/item/20-01-2021-who-information-notice-for-ivd-users-2020
February 12, 2021
CSF Leak After COVID-19 Nasopharyngeal Swab: A Case Report
iatrogenic CSF leak following a nasal swab
January 21, 2021
European Journal of Neurology
Meningitis due to cerebrospinal fluid leak after nasal swab testing for COVID-19
January 14, 2021
Journal of Clinical Neuroscience
CSF rhinorrhoea post COVID-19 swab: A case report and review of literature.
“With most of commercially available swabs exceeding these dimensions (Fig. 3 A) improperly directed swabs can cause mucosal damage as well potential damage to the skull base.”
December 31, 2020
The criminal WHO blows its own cover: fake PCR test / Explanation / Translation of the WHO Notice
https://blog.nomorefakenews.com/2020/12/31/the-criminal-who-blows-its-own-cover-fake-pcr-test/
The PCR test is virtually THE foundation of the Covid narrative.
Without it you have nothing but healthy people and the normal winter flulike illnesses. Every ‘case’ you read about is only a case because of a PCR test.
We and others have been saying since at least June that the PCR test is scientifically meaningless. And now, the WHO is admitting it too.
And if the PCR test is meaningless. So is the “pandemic”.
January 11, 2021
Corman Drosten Addendum
The global team of scientists and experts wrote another 60 page dissection of the Drosten PCR protocol. Answering criticism of the initial retraction request not having enough “wet-lab” proof.
20 peer reviewed papers showing catastrophic problems.
https://cormandrostenreview.com/addendum/
“We believe the references provided in this addendum itemize the scientific consensus evident in the literature regarding the flaws in the original PCR detection method for SARs-CoV-2 published by Corman et al.. Further, since several important flaws were published in peer-reviewed journals, the lack of correction of the original PCR protocol by either Eurosurveillance or as an update in the Charité-WHO protocol brings into question the scientific integrity of the authors of Corman et al. These references settle any remaining debate that the Corman et al. manuscript should be retracted on technical grounds alone. The rapidity of the peer-review and conflicts of interest are even more troubling.”
– Corman Drosten Addendum
December 14, 2020
In a statement released on December 14, 2020 the World Health Organization finally owned up to what 100,000’s of doctors and medical professionals have been saying for months: the PCR test used to diagnose COVID-19 is a hit and miss process with way too many false positives.
https://www.who.int/news/item/14-12-2020-who-information-notice-for-ivd-users
December 13, 2020
A brief history of the PCR fiasco of the Pandemic that can’t seem to end.
https://threadreaderapp.com/thread/1338171321637810178.html
December 13, 2020
SARS-CoV-2 RNA reverse-transcribed and integrated into the human genome
We investigated the possibility that SARS-CoV-2 RNAs can be reverse-transcribed and integrated into the human genome and that transcription of the integrated sequences might account for PCR-positive tests.
https://www.biorxiv.org/content/10.1101/2020.12.12.422516v1.full
December 3, 2020
Viral cultures for COVID-19 infectious potential assessment – a systematic review
Those with high cycle threshold are unlikely to have infectious potential.
https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa1764/6018217
November 28, 2020
Retraction request letter to Eurosurveillance editorial board
https://cormandrostenreview.com/retraction-request-letter-to-eurosurveillance-editorial-board/
November 27, 2020
Corman Drosten Review Report
A global team of experts has found 10 FATAL FLAWS in the main test for Covid and is demanding it’s urgently axed.
External peer review of the RTPCR test to detect SARS-CoV-2 reveals 10 major scientific flaws at the molecular and methodological level: consequences for false positive results.
In light of our re-examination of the test protocol to identify SARS-CoV-2 described in the Corman-Drosten paper we have identified concerning errors and inherent fallacies which render the SARS-CoV-2 PCR test useless.
https://cormandrostenreview.com/report/
“In light of our re-examination of the test protocol to identify SARS-CoV-2 described in the Corman-Drosten paper we have identified concerning errors and inherent fallacies which render the SARS-CoV-2 PCR test useless.”
– Corman Drosten Review Report
November 26, 2020
The PCR FalsePositive Pseudo-Epidemic
A “case” is a positive PCR test. No symptoms are involved. A “COVID-19 admission” to a hospital is a person testing positive by PCR before, on entry or at any time during a hospital stay, no matter the reason for the admission or the symptoms the patient is presenting. A “COVID-19 death” is any death within 28 days of a positive PCR test. If there is any doubt about the reliability of the PCR test, all of this falls away at a single stroke.
In summary, I argue that it is criminally dangerous to drive policy based in any way on this test (set up the way it is) and its results. No amount of argument or prevarication can alter these damning facts.
PCR-based COVID testing has failed and is not a proper basis to
lockdown the nation, let alone decide on tiers for restrictions
https://lockdownsceptics.org/wp-content/uploads/2020/11/MP-briefing-26-Nov-2020.pdf
November 18, 2020
In November 2020 judges in Lisbon have described the reliability of tests being rolled out in their tens of thousands as “more than debatable”.
A 34-page ruling on an appeal against a writ of habeas corpus filed by four German tourists …leaves no doubt that a positive RT-PCR test cannot be taken on face value.
https://www.portugalresident.com/judges-in-portugal-highlight-more-than-debatable-reliability-of-covid-tests/
https://greatgameindia.com/portuguese-court-pcr-tests-unreliable/
November 10, 2020
PCR “Pandemic”, Cycle Thresholds of 35+ = False
Positives, Even Tony Fauci Says So!
https://www.youtube.com/watch?v=QJfvnzGBqoA&feature=emb_logo
with Emeritus Prof. Beda Stadler, Prof Carl Heneghan, Dr. Fauci, Kary Mullins, Professor Stephen A. Bustin
October 1, 2020
Cerebrospinal Fluid Leak After Nasal Swab Testing for Coronavirus Disease 2019
“This case of iatrogenic CSF leak from nasal swab testing for COVID-19 illustrates that prior surgical intervention, or pathology that distorts normal nasal anatomy, may increase the risk of adverse events associated with nasal testing for respiratory pathogens, including COVID-19.”
September 28, 2020
Correlation Between 3790 Quantitative Polymerase Chain Reaction–Positives Samples and Positive Cell Cultures, Including 1941 Severe Acute Respiratory Syndrome Coronavirus 2 Isolates
The 👆🏼 Portuguese judges cited the following study conducted by “some of the leading European and world specialists
https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa1491/5912603
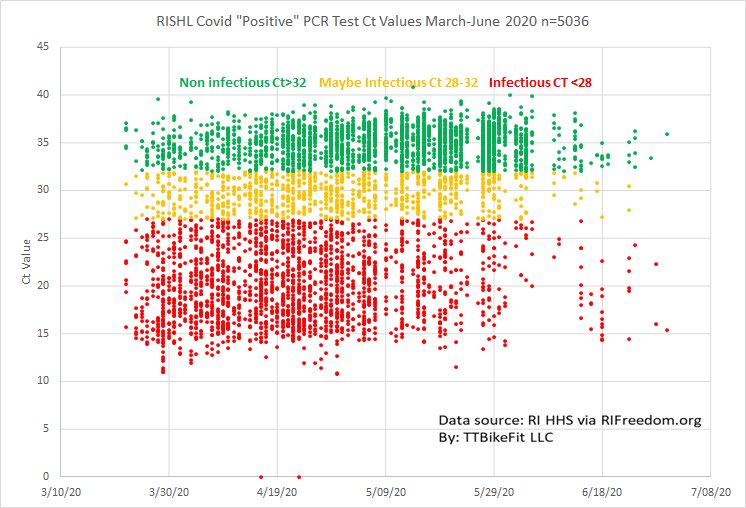
“At a cycle threshold (ct) of 25, about 70% of samples remain positive in cell culture (i.e. were infected): in a ct of 30, 20% of samples remained positive; in a ct of 35, 3% of samples remained positive and in a ct above 35, no sample remained positive (infectious) in the culture”.
“This means that if a person has a positive PCR test at a threshold of cycles of 35 or higher (as happens in most laboratories in the USA and Europe), the chances of a person being infected is less than 3%. The probability of a person receiving a false positive is 97% or higher”.
September 22, 2020
The polymerase chain reaction (PCR) swab testis useful (but not perfect) for detecting SARS-CoV-2 virus RNA in symptomatic patients. However, problems arise using the test for purposes that disregard symptoms or time of infection—namely, case finding, mass screening, and disease surveillance. This is because PCR is not a test of infectiousness.
https://www.bmj.com/content/370/bmj.m3699.short?rss=1&utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+bmj%2Frecent+%28Latest+from+BMJ%29
The FDA did not regularly approve the PCR test, but approved it “For Emergency Use Only”.
“The CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel is only for use under a Food and Drug Administration’s Emergency Use Authorization. “
FDA also states:
“Positive results are indicative of active infection with
SARS-CoV-2 but do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.”
https://www.fda.gov/media/134922/download
April 2020
In April 2020, a laboratory in Augsburg, Germany published the following information on their website:
Quote:
“Modified reporting layout of SARS-Cov-2 PCR results.
From now on, we will only print the result as positive or negative on our findings.
Previously, you received two results depending on the test used.
If the sample was analyzed using the Roche method, we have reported the test results for both target sequences of the PCR (ORF1 and E gene) separately.
The ORF1 gene is specific for SARS-CoV-2, whereas the E gene is also present in other coronaviruses. The cases in which only the ORF gene was amplified, have also been positively assessed by us before. Few cases with isolated positive E gene were considered questionable and therefore repeatedly led to queries and problems regarding further management of affected patients. Taking into account the epidemiological situation and the overall increase in the positive rate, from now on we follow the WHO recommendation and report a result as “positive” when just the E gene has been amplified. Therefore, to simplify the findings, only one overall result (positive or negative) will appear in the future. A result is positive if at least one of the two target sequences of SARS-CoV-2 was detected in the swab material.
If the sample was analyzed using methods from rBiopharm or TibMolbiol, we have previously performed separate screening and confirmatory tests. Analogous to the approach described above, we restrict ourselves to the previous screening test targeting the E gene because of its high positive predictive value with increasing COVID-19 prevalence.”
End of Quote.
A day later, this notification was removed from the website.
The Invented Pandemic, the Lack of Virus Isolation and the Invalid COVID-19 Test.
Fauci knew that the PCR Tests is done by to high cycles above 35 (which are fake positives) in Juli 2020
Kary Mullins PCR Inventor:
“PCR is just a process..it does not tell you that you are sick.”
“With PCR, if you do it well, you can find almost anything in anybody.”
Dr Karyy Mullins – Inventor of the PCR test and Nobel Prize winning scientist
Extended interview
Interview
January 23, 2021
Return to Wuhan: What Life Is Like One Year Later / NBC Nightly News
Dr. Wu Zunyou / Chinese Center For Disease Control
“They didn’t isolate the virus. That’s the issue.”
Correlation Between 3790 Quantitative Polymerase Chain Reaction–Positives Samples and Positive Cell Cultures
Non-infectious/ Maybe infectious/ Infectious
Drosten Protocol paper
Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR
https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.3.2000045
WHO – Drosten Protocol
Diagnostic detection of 2019-nCoV by real-time RT-PCR
CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel
For Emergency Use Only
Instructions for Use
Virology, transmission, and pathogenesis of SARS-CoV-2
https://www.bmj.com/content/371/bmj.m3862
Reverse transcription polymerase chain reaction (RT-PCR) tests can detect viral SARS-CoV-2 RNA in the upper respiratory tract for a mean of 17 days; however, detection of viral RNA does not necessarily equate to infectiousness, and viral culture from PCR positive upper respiratory tract samples has been rarely positive beyond nine days of illness
Symptomatic and pre-symptomatic transmission (1-2 days before symptom onset), is likely to play a greater role in the spread of SARS-CoV-2 than asymptomatic transmission
A wide range of virus-neutralising antibodies have been reported, and emerging evidence suggests that these may correlate with severity of illness but wane over time
- Most clinical presentations are mild, and the typical pattern of covid-19 more resembles an influenza-like illness—which includes fever, cough, malaise, myalgia, headache, and taste and smell disturbance—rather than severe pneumonia
bioPerfectus technologies
COVID-19 Coronavirus Real Time PCR Kit
“Positive result suggests SARS-CoV-2 infection but bacteria and other virus induced co-infection could not be excluded. SARS-CoV-2 test result is not the only confirmation evidence of suspected cases and all positive results have to be reported to Centers for Disease Control (CDCs) and authorities.”
https://www.who.int/diagnostics_laboratory/eual/eul_0515_202_00_covid19_coronavirus_real_time_pcr_kit_ifu.pdf?ua=1
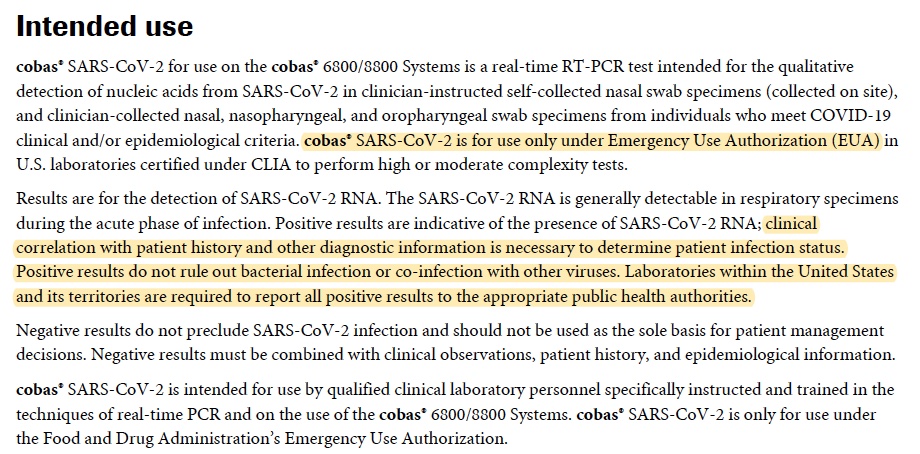
cobas® SARS-CoV-2 Test
Hoffmann-La Roche
– This test has not been FDA cleared or approved
– Clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses.
Kary Mullins about Anthony Fauci

– Kary Mullins
Jens Spahn, German Minister of Health
“We now have to be careful that we don’t have too many false positives due to too much testing.”
We urgently need your support !!
There are many easy ways how to do this. Please find the information here:
>How to Support Us <
>Shop at our Affiliate Partner Shops<
Find books, nutritions, vitamins, groceries, outdoor products, travel deals, and many more on our pages:
Dietary Supplements, Immune Support
or please Donate here 🙂
All information is deemed accurate but not guaranteed and should be independently verified.